Received: Thu 28, Nov 2024
Accepted: Fri 20, Dec 2024
Abstract
Purpose: The aim of this study was to assess the oncologic outcome of pelvic bone sarcomas (PBS) and to identify prognosis factors.
Patients and Methods: We report a multicentric cohort of patients treated for a PBS from 2000 to 2020. Data from 12 hospitals were analysed. Patients treated for primary PBS were included. Alive patients with less than 6 months of follow up were excluded. The primary outcome was survival.
Results: One hundred and fourteen patients (67 males and 48 females) were reviewed with a mean follow up of 32±46.5 (1 to 216) months. The mean patient and doctor diagnosis delays were respectively 8.5±10.2 (1 to 60) and 3±4.3 (0 to 24) months. Sixty-eight patients (59.6%) died after a mean time from diagnosis of 15.9±22.8 (1 to 120) months. The overall survival rates at 5 and 10 years were respectively 38.4% and 27.6%. Chondrosarcoma histological type (HR=3.64), metastasis (HR=3.55) and surgery (HR=0.12) were identified as significant survival factors. Surgery was also associated to a decreased risk of metastasis (OR=0.03, 95% CI: 0.01 - 0.1). Among the 76 patients (66.7%) who underwent surgery, local recurrence was observed in 19 patients (25%) with a mean time from surgery to onset of 11.05 (±17.5) months.
Conclusion: This nation-wide 20-year-cohort study shows that surgery is the most effective treatment option in PBS regardless the histological type of the tumour. Efforts have to be done to decrease the diagnosis delay in order to start treatment when surgery is still feasible.
Keywords
Osteosarcoma, chondrosarcoma, Ewing sarcoma, pelvis, surgery, prognosis
1. Introduction
The pelvis is a relatively rare location for malignant primary bone tumours. It’s however one of the most challenging problems in orthopaedic oncology [1, 2]. Deep location of the tumour is frequently responsible of a delay in diagnosis. Bone sarcoma of the pelvis is also characterized by a poor outcome compared to those of the extremities. Reasons include huge mass and distant metastases at presentation, difficulties to achieve safe margins and a poorer response to chemotherapy (CT). Surgery is the backbone of the treatment. It’s frequently associated to CT and/or radiotherapy (RT). Indications depend mainly on histological type of the tumour and its stage. When surgery is feasible, limb salvage is the preferred option. It’s however a high demanding procedure with high morbidity [2, 3].
The objectives of this retrospective study were to assess the oncological outcome of bone sarcomas of the pelvis and to determine their prognostic factors.
2. Patients and Methods
We performed a retrospective study analysing data from 12 public and private (country name occulted for peer review) hospitals. Inclusion criteria were primary malignant bone tumours involving the pelvis treated between 2000 and 2020. Medical records included the age and gender of the patient, duration and description of the symptoms, imaging results and histological diagnosis. Tumour location was assigned according to surgical areas defined by Enneking and Dunham [1]. Tumours were staged at the time of diagnosis according to the staging system for bone sarcomas described by Enneking [4]. All patients were followed at least for 6 months or until death. Postoperative complications were recorded and functional outcome was assessed using the musculoskeletal tumour society (MSTS) functional rating system [5]. Oncologic results were evaluated according to local recurrence, metastasis, or death. Survival was defined as the time from diagnosis to last follow-up or death from any cause.
Were excluded from this study: bone metastases, benign tumours, chordomas, soft tissue sarcomas and tumours originating from the sacrum. Were excluded also patients with lost data and those who were alive and with a follow up less than 6 months.
2.1. Statistical Analysis
Descriptive analysis was made using median values and 95% confidence interval (CI). Differences between groups were assessed using the Chi-squared test. For statistical analysis, overall survival was calculated according to the Kaplan-Meier method. To determine prognostic factors, survival curves were compared in a univariate analysis using the log-rank test. A p-value of less than 0.05 was considered significant. Factors with p-value < 0.25 were introduced into a multivariate analysis using the Cox model or binary logistic regression. All statistical analyses were carried out using the SPSS software version 23.0 (SPSS, Inc., Chicago, IL).
3. Results
3.1. Epidemiology
One hundred and fourteen patients fulfilled all criteria and were enrolled into this study. There were 67 males and 48 females. The mean age was 30.1 years. Twenty-five patients (21.9%) were aged below 18. The mean patient and doctor delays were respectively 8.5 ±10.2 (1 to 60) and 3 ±4.3 months (0 to 24). The longest patient delay was observed in CS (p=0.001). Pelvic pain was the main symptom reported 89.5% of cases, however swelling was present in half of the cases only. Tumour was located in the PI+/-IV in 67 patients (58%) and PII was involved in 39 cases (34.2%). Three histological types were diagnosed: chondrosarcoma (CS), Ewing sarcoma (ES) and osteosarcoma (OS). eighty-one tumours (71%) were localized and 33 (29%) were metastatic. Epidemiologic data are summarized in (Table 1).
3.2. Treatment
Surgery was performed in 76 cases (66.7%). It concerned 86.4% of the non-metastatic patients (70 cases) and only 18.2% of the metastatic ones (6 cases) (p<0.0001). Internal hemipelvectomy was performed in 69 cases (90.5%). In four cases, the surgery was external hemipelvectomy. In three patients an intra-lesional curettage was done as a debulking procedure.
In PI+/-IV resections (37 cases) bone defect was reconstructed in 35 cases using free fibula grafting and in 2 cases we used bone cement and plate. In PI+II (9 cases) it was done sacro-femoral arthrodesis in 7 cases and ischio-femoral arthrodesis in 2 cases. In PII+III resections (6 cases), ilio-femoral (IF) arthrodesis was performed. Total hemipelvectomy (PI/II/III) was performed in 4 cases. Reconstruction was performed in 1 case using bone cement and total hip arthroplasty. In 3 cases, there was no reconstruction. In PII resections (2 cases) reconstruction was performed only in one case by IF arthrodesis. PIII (4 cases) and partial PI resections (7 cases) were not reconstructed. Surgical margins were R0 in 60 cases (79%) R1 in 13 cases (17%) and R2 in 3 cases (4%). Post-operatively, major complications were reported in 8 patients. Wound necrosis and deep infection occurred in 4 cases. They were treated by surgical debridement and antibiotics in 3 cases. In one case of reconstruction with cement and arthroplasty, septic dislocation of the hip occurred and needed secondary hindquarter amputation. Sciatic palsy had occurred in 4 cases. None of the reconstructions was revised for mechanical failure.
TABLE 1. Characteristics of our
population.
|
CHARACTERISTICS |
TOTAL PATIENTS Nb(%) |
HISTOLOGICAL TYPE |
||
|
CS |
Ew |
OS |
||
|
Size (n (%)) |
114 (100) |
49 (43) |
40 (35.1) |
25 (21.9) |
|
Sex |
|
|
|
|
|
Male (n
(%)) |
67(58.8) |
|
|
|
|
Female (n
(%)) |
47(41.2) |
|
|
|
|
Age Mean
(years)+/- SD ( Range) |
30.2 +/- 14.7 (6-72) |
37.6 +/- 11.8 |
17.9 +/- 7 |
35.3 +/- 16.4 |
|
Age <=17
(n (%)) |
25 (21.9) |
0 |
22 (55) |
3 (12) |
|
Age >17
(n (%)) |
89 (78.1) |
49 |
18 (45) |
22 (88) |
|
Patient delay (month) |
8.5 |
13 |
4.81 |
5.7 |
|
Symptoms Pain (n
(%)) Swelling (n
(%))
Neurological signs (n (%)) Other (n
(%)) |
100 (87.7) 59 (51.8) 15 (13.1) 15 (12) |
|
|
|
|
Enneking zoning I+/-IV (n
(%)) II (n (%)) III (n (%)) I+II (n
(%)) II+III (n
(%)) I+II+III (n
(%)) |
67 (58.7) 7 (6.1) 8 (7) 12 (10.5) 9 (7.9) 11 (9.6) |
|
|
|
|
Staging IB (n (%)) IIB (n (%)) III (n (%)) |
13 (11.4) 68 (59.6) 33 (28.9) |
13 (26.5) 26 (53.1) 10 (20.4) |
0 27 (67.5) 13 (32.5) |
0 15 (60) 10 (40) |
|
Treatment Surgery
Chemotherapy
Neoadjuvant
adjuvant
Combined to RT
Palliative
Radiotherapy |
76(66.7) 77(67.5) 42(54.5) 2(2.6) 9(11.6) 24(31.1) 19(16.7) |
37(75.5) 14 (28.6) 3(6.1) 0 3(6.1) 8(16) 4(8.2) |
28(70) 39(97.5) 31(77.5) 0 2(5) 6(15) 11(27.5) |
11(44) 24(96) 8(32) 2 4(16) 10(40) 4(16) |
Chemotherapy was used in 77 cases (67.5%) mostly in ES and OS. It was associated to surgery in 44 cases (57.1%), it was combined to RT as definitive treatment in 9 cases and was used in a palliative attempt in 24 cases (18 of them were metastatic). Histological response was available in 33 patients who had neoadjuvant CT (23 Ew, 8 OS and 2 CS). Good response was observed in 82% of ES. Seven patients with OS (87.5%) and both patients with CS had poor response (p<0.001). Radiotherapy was used in 19 patients (16.7%) among them 11 patients had ES, 11 were metastatic and only 5 patients had had surgery (detailed therapeutic protocols are reported in Table 1).
According to metastatic status, there were more surgeries in non-metastatic patients (p < 0.001) and more RT in metastatic ones (p = 0.002).
TABLE 2.
Prognostic factors for oncological outcome.
|
|
5 YSR % p |
LR* Nb/% p |
Mets** Nb/% p |
|
Margins R0
R1/R2 |
66.6 0 <0.001 |
6/10.2 13/81.3 <0.001 |
15/26.8 9/64.2 0.001 |
|
Histology CS
Ew
OS |
59.7 23.5 0.01 19.6 |
8/21.6 8/28.6 ns 3/30 |
12/30.8 13/48.1 ns 8/ 53.3 |
|
Surgery Yes No |
56.9 3.9 <0.0001 |
- |
24/34.3 9/81.8 0.003 |
|
Stage III Yes No |
0 53.5 <0.0001 |
- |
- |
|
Nb of zone
1
>1
|
47.3 25.4 ns |
10/19.2 9/39.1 0.06 |
17/32.1 11/52.1 ns |
|
Response to CT Good (20) Poor
(12) |
33.3 20.9 0.02 |
6/30 7/58.3 0.006 |
7/43.8 10/71.4 0.02 |
|
Age <= 17 > 17 |
43.7 36.1 ns |
- |
- |
*LR was determined in patients who had surgery.
**Metastasis were determined in non-metastatic patients at presentation.
TABLE 3. Prognostic factor for OS in univariate and multivariate analysis.
|
|
Univariate
analysis Brut HR (95
% CI) |
Multivariate
analysis Adjusted HR
|
||||
|
|
HR |
95%CI |
p |
HR |
95%CI |
p |
|
Histological type |
|
|||||
|
CS |
Ref |
|
|
Ref |
|
|
|
Ew |
2.39 |
[1.34
-4.26] |
0.03 |
2.84 |
[1.18 - 6.80] |
0.01 |
|
OS |
2.98 |
[1.58
-5.62] |
0.01 |
3.64 |
[1.23 – 10.73] |
0.01 |
|
Response to CT |
|
|
|
|
|
|
|
Good |
Ref |
|
|
|
|
|
|
Poor |
1.59 |
[0.77 -
3.25] |
0.349 |
|
|
|
|
Number of zones |
|
|
|
|
|
|
|
1 |
Ref |
|
|
Ref |
|
|
|
> 1 |
1.44 |
[0.87-2.41] |
0.15 |
1.22 |
[0.58–2.58] |
0.58 |
|
Surgery |
|
|
|
|
|
|
|
No |
Ref |
|
|
Ref |
|
|
|
Yes |
0.19 |
[0.11-
0.32] |
0.001
|
0.12 |
[0.03 – 0.42] |
0.001 |
|
Metastasis |
|
|
|
|
|
|
|
No |
Ref |
|
|
Ref |
|
|
|
Yes |
4.38 |
[2.64 –
7.27] |
0.001
|
3.55 |
[1.28 – 9.48] |
0.01 |
HR: Hazard Ratio, (95 % CI) : confidence interval at 95%.
3.3. Oncologic Results (Tables 2 & 3)
Patients were reviewed with a mean FU of 32 months (SD: 46.5 - range: 1 to 216). At the last FU, 68 patients (59.6%) died after a mean time from the diagnosis of 15.9 months (SD: 22.8 - range: 1 to 120). All of them but one died from disease progression. Forty-six patients (40.4%) were alive with a mean FU of 56 months (SD: 60.6 - range: 6 to 216). Among them, 42 were disease free.
3.4. Local Recurrence
Local recurrence (LR) was observed in 19 patients (25%). The mean time from surgery to onset of LR was 11.05 months (SD 17.6 - range: 1 to 60). In univariate analysis, only inadequate surgical margins (R1 or R2) and poor response to CT were significantly associated to a high risk of LR. Resection of more than one zone was also associated to higher rate of LR but was not significant. The multivariate analysis showed that only surgical margins were independently associated to tumour recurrence (HR = 8.16, p=0.01, CI=2.29 - 29.03).
3.5. Metastasis
Among the 81 localized tumours at presentation, 33 patients (40.7%) presented lung metastasis at the last follow up. In univariate analysis, inadequate surgical margins, poor response to CT and patients managed without surgery were significantly associated to a higher risk of metastases. Multivariate analysis showed that surgery was associated with a decreased risk of metastasis with an OR of: 0.03 95% CI [0.01 - 0.10].

Mets: Metastasis at Presentation.
3.6. Survival
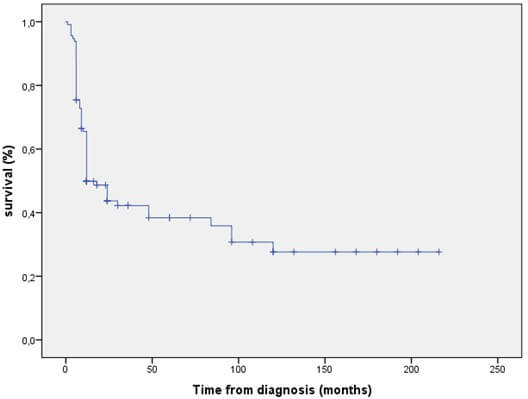
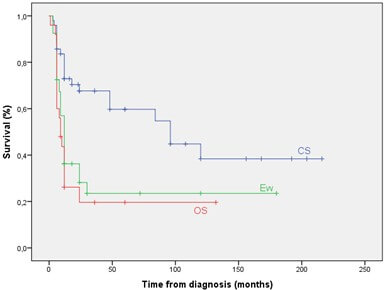
The overall survival rate at 5 and 10 years were 38.4 % and 27.6 % respectively (Figure 1). The 5YOS was significantly better in non-metastatic patients (Figure 2), in patients with CS (Figure 3), when patients were treated by surgery (Figure 4), when surgical margins were safe and in good responder patients. In multivariate analysis, significant prognostic factors for death were histological type, the metastatic status and surgery. OS and metastasis increased the risk of death with a HR of 3.64 and 3.55 respectively. On the other hand, surgery was associated with a decreased risk of death with a HR of 0.12.

3.7. Anatomical and Functional Outcome
Forty patients had biological reconstruction. At the last FU, 19 were evaluated and in all of them we obtained bone healing. Functional score was evaluated in 33 patients. The mean MSTS score was 26.27/30 (range 15 to 30). Patients with partial PI resection (7 cases) had the highest score (30/30) and those with PI/II/III resection (2 cases) had the lowest score (mean 20/30). Patients with PI resection (6 cases) and those with IF arthrodesis (3 cases) had a mean score respectively of 26.67/30 and 24/30.
4. Discussion
Our study highlighted the different challenges in the management of bone sarcoma of the pelvis. The first difficulty is the delay in the diagnosis. Symptoms are often insidious and nonspecific mimicking those of more commonly encountered non-neoplastic musculoskeletal conditions [6, 7]. Plain radiographs have a poor sensibility and usually fail to show subtle and small size images. Lesions are easier to identify when located on PIII. In addition, overlying digestive gas could occult lytic images [8]. Facing a persistent inflammatory pain and/or compression symptoms with no clear explanation on plain radiographs, it is recommended to perform a cross-sectional imaging [9, 10].
Therapeutic management of pelvic sarcomas involves a multidisciplinary team and should be performed in referral centres. Chemotherapy has large indications in bone sarcoma of the pelvis and is generally integrated in a multimodal approach. Indications depend mainly on histotype of the tumour. ES is the most sensitive to CT. Neoadjuvant protocols lead in most cases to a downstaging of the lesion allowing limb salvage with safe margins as observed in our series (82% of good responders). However, pelvic OS are less sensitive to CT (only 19% good responders in our series). These findings are consistent with similar studies. Xu et al. [11] and Ferrari et al. [12] reported that only 8% and 24% of their patients respectively had a necrosis rate superior to 90%. One of the reasons is that pelvic OS are mainly chondroblastic. These results made the clinical relevance of neoadjuvant CT in pelvic OS debatable. Xu et al. [11] examined the timing of CT in pelvic osteosarcoma and found that neoadjuvant CT did not alter overall survival or local recurrence compared to early surgery.
As for CT, ES is very sensitive to RT. In our study, RT was clearly underused in localized and metastatic tumours as well. Classically, radiation is indicated after surgery in poor responders and in case of positive margins [13]. Current studies recommend considering RT even in case of negative margins [14]. RT remains the only options combined to CT in unrespectable tumours [15]. Whether surgery is superior to RT for local control of ES or not is also debatable. Several studies showed 5-year local control ranging from 70% to 75% by using RT alone in ES [13, 16, 17].
Surgery is the backbone of the treatment of pelvic sarcomas. It’s however less frequently performed compared to extremity tumours due to the advanced stage of the tumours at presentation, the complex anatomy of the pelvis and the higher complications rate [18-21]. Only two third of our patients underwent surgery. Primary goal of surgery is to achieve safe margins and the secondary goal is preservation of limb function. The rate of safe margins for pelvic sarcoma in the literature varies largely between 25 and 82% [22, 23]. It was 78.9% in our study. Recently, new surgical techniques have been developed to improve resection accuracy, including computed tomography, guided navigation with an O-arm, optical navigation and patient-specific instruments [24].
Since the 1980s, limb salvage procedures have replaced amputation thanks to the introduction of effective CT and the advancement of imaging and surgical techniques [25]. In our study, 90% of patients had internal hemipelvectomy. This procedure needs usually reconstruction which depends on the type of resection. In type I with interruption of the pelvic ring continuity, reconstruction is mandatory [26]. The use of double-barrelled fibula autograft gives good clinical and functional outcome. Reconstruction after periacetabular tumour resection is however much more difficult [27]. Various reconstruction methods have been described including autograft with recycled bone, allograft and custom-made or modular pelvic prostheses [28, 29]. These procedures are associated with high rate of infection and mechanical failure [30]. In our experience, we have favoured arthrodesis in PII+III resections. Despite the loss of hip function and leg length discrepancy this procedure has the advantages to be a definitive reconstruction with very limited morbidity. PI+II resections are the most challenging and the question of whether reconstruction in these cases is justified remains unanswered [31-33]. Some authors recommend restoring the anatomy in order to avoid flail hip [34]. However, for others these procedures are associated with high rates of infection, mechanical failure and poor functional result [35]. We believe that no reconstruction is a reliable option for such patients with limited life expectancy and who need quick recovery to continue their therapeutic protocol.
There is a general consensus in the literature regarding the poor outcomes of sarcoma involving the pelvic bones [36-39]. Jawad et al. [39] analysed the largest series of 1185 pelvic sarcoma cases (including 18.3% chordomas) from the surveillance, epidemiology, and end results database from 1987 to 2006. The 5YOS for all the patients with pelvic sarcoma was 45% (59% in CS, 46% in ES, and 19% in OS). Stage of the tumour is the second most important predictor factor for survivor [36, 37, 39]. Other factors reported in the literature included age, size of the tumour, and use of surgical treatment [39]. These results are consistent with our finding especially for histologic type, the stage and surgical treatment.
Factors influencing the local control of the disease include quality of surgical margin and tumour volume [36-39]. Indeed, in our series positive margins and poor response to CT were significantly associated to a high risk of local recurrence. Volume of the tumour - assimilated in this study to the extension of the resection - showed higher rate of LR when the resection included more than one zone (19.2% vs 39.1%) however this was not significant.
In summary, to improve outcome of pelvic sarcomas efforts have to be done for an early diagnosis of the lesions. As for all the musculoskeletal pathology, patients should be integrated in a multidisciplinary approach and managed in referral centres. Surgery is the most effective treatment regardless the histotype of the tumour. It is associated to CT and RT in ES. In patients with OS, the best timing of CT is still unknown. Reconstruction of the pelvis should avoid complex procedures which are associated to a high complications rate and poor functional outcome.
Funding
None.
Conflicts of Interest
None.
Availability of Data and Material
Available under request from authors.
Code Availability
Not applicable.
Author Contributions
All authors contributed in the collection and analysis of data, in writing and reviewing the manuscript.
Ethics Approval
Data was collected and analysed in respect of national and international legislation about personal data protection.
Consent to Participate
Not applicable.
Consent for Publication
All authors give their approval for publication of the manuscript.
REFERENCES
1.
W F Enneking, W K Dunham “Resection and reconstruction
for primary neoplasms involving the innominate bone.” J Bone Joint Surg Am,
vol. 60, no. 6, pp. 731-746, 1978. View at: PubMed
2.
M I O'Connor, F H Sim “Salvage of the limb in the
treatment of malignant pelvic tumors.” J Bone Joint Surg Am, vol. 71,
no. 4, pp. 481-494, 1989. View at: PubMed
3.
Andrea Angelini, Teresa Calabrò, Elisa Pala, et al.
“Resection and Reconstruction of Pelvic Bone Tumors.” Orthopedics, vol. 38, no.
2, pp. 87-93, 2015. View at: Publisher
Site | PubMed
4.
W F Enneking “A system of staging musculoskeletal
neoplasms.” Clin Orthop Relat Res, no. 204, pp. 9-24, 1986. View at: PubMed
5.
W F Enneking, W Dunham, M C Gebhardt, et al. “A system
for the functional evaluation of reconstructive procedures after surgical
treatment of tumors of the musculoskeletal system.” Clin Orthop Relat Res,
no. 286, pp. 241-246, 1993. View at: PubMed
6.
Johan L Bloem, Inge I Reidsma “Bone and soft tissue
tumors of hip and pelvis.” Eur J Radiol, vol. 81, no. 12, pp. 3793-3801,
2012. View at: Publisher Site | PubMed
7.
L D Wurtz, T D Peabody, M A Simon “Delay in the
diagnosis and treatment of primary bone sarcoma of the pelvis.” J Bone Joint
Surg Am, vol. 81, no. 3, pp. 317-325, 1999. View at: Publisher
Site | PubMed
8.
Prabhakar Rajiah, Hakan Ilaslan, Murali Sundaram
“Imaging of Sarcomas of Pelvic Bones.” Seminars in Ultrasound, CT and MRI,
vol. 32, no. 5, pp. 433-441, 2011. View at: Publisher Site | PubMed
9.
Kumar R, Kumar M, Malhotra K, Patel S. Primary
Osteosarcoma in the Elderly Revisited: Current Concepts in Diagnosis and
Treatment. Curr Oncol Rep [Internet]. 2018;20(2):20-33. Available from:
http://dx.doi.org/10.1007/s11912-018-0658-1 View at: Publisher
Site
10. P.G. Casali, S.
Bielack, N. Abecassis, et al. “Bone sarcomas: ESMO-PaedCan-EURACAN Clinical
Practice Guidelines for diagnosis, treatment and follow-up.” Ann Oncol,
vol. 29, pp. iv79- iv 95, 2018. View at: Publisher Site
11. Jie Xu, Lu Xie,
Wei Guo “Neoadjuvant Chemotherapy Followed by Delayed Surgery: Is it Necessary
for All Patients With Nonmetastatic High-Grade Pelvic Osteosarcoma?” Clin
Orthop Relat Res, vol. 476, no. 11, pp. 2177-2186, 2018. View at: Publisher
Site | PubMed
12. Stefano Ferrari, Emanuela Palmerini,
Nicola Fabbri, et al. “Osteosarcoma of the pelvis: a monoinstitutional
experience in patients younger than 41 years.” Tumori, vol. 98, no. 6,
pp. 702-708, 2012. View at: Publisher
Site | PubMed
13. Torunn I Yock,
Mark Krailo, Christopher J Fryer, et al. “Local control in pelvic Ewing
sarcoma: analysis from INT-0091--a report from Children’s Oncology Group.” J
Clin Oncol, vol. 24, no. 24, pp. 3838-3843, 2006. View at: Publisher
Site | PubMed
14. Nacional
Comprehensive Cancer Network Guidelines Version 1.2021.
15. Julia Haeusler, Andreas Ranft, Tobias
Boelling, et al. “The value of local treatment in patients with primary, disseminated,
multifocal Ewing sarcoma (PDMES).” Cancer, vol. 116, no. 2, pp. 443-450,
2010. View at: Publisher Site | PubMed
16. J Dunst, H Jürgens, R Sauer, et al. “Radiation
therapy in Ewing’s sarcoma: an update of the CESS 86 trial.” Int J Radiat
Oncol Biol Phys, vol. 32, no. 4, pp. 919-930, 1995. View at: Publisher
Site | PubMed
17. Daniel J
Indelicato, Sameer R Keole, Amir H Shahlaee, et al. “Definitive radiotherapy
for ewing tumors of extremities and pelvis: long-term disease control, limb
function, and treatment toxicity.” Int J Radiat Oncol Biol Phys, vol.
72, no. 3, pp. 871-877, 2008. View at: Publisher
Site | PubMed
18. D Donati, S
Giacomini, E Gozzi, et al. “Osteosarcoma of the pelvis.” Eur J Surg Oncol,
vol. 30, no. 3, pp. 332-340, 2004. View at: Publisher
Site | PubMed
19. Raya Saab,
Bhaskar N Rao, Carlos Rodriguez-Galindo, et al. “Osteosarcoma of the pelvis in
children and young adults: the St. Jude Children’s Research Hospital
experience.” Cancer, vol. 103, no. 7, pp. 1468-1474, 2005. View at: Publisher Site | PubMed
20. Stefano
Ferrari, Emanuela Palmerini, Nicola Fabbri, et al. “Osteosarcoma of the pelvis:
a monoinstitutional experience in patients younger than 41 years.” Tumori,
vol. 98, no. 6, pp. 702-708, 2012. View at: Publisher
Site | PubMed
21. Michael S
Isakoff, Donald A Barkauskas, David Ebb, et al. “Poor Survival for Osteosarcoma
of the Pelvis A Report from the Children’s Oncology Group.” Clin Orthop
Relat Res, vol. 470, no. 7, pp. 2007-2013, 2012. View at: Publisher Site |
PubMed
22. M I O'Connor, F
H Sim “Salvage of the limb in the treatment of malignant pelvic tumors.” J
Bone Joint Surg Am, vol. 71, no. 4, pp. 481-494, 1989. View at: PubMed
23. G L Farfalli, J I Albergo, L E Ritacco,
et al. “Oncologic
and clinical outcomes in pelvic primary bone sarcomas treated with limb salvage
surgery.” Muskuloskelet Surg, vol. 99, no. 3, pp. 237-242, 2015. View
at: Publisher Site | PubMed
24. Robin Evrard, Thomas Schubert, Laurent
Paul, et al. “Resection
margins obtained with patient-specific instruments for resecting primary pelvic
bone sarcomas: A case-control study.” Orthop Traumatol Surg Res, vol.
105, no. 4, pp. 781-787, 2019. View at: Publisher
Site | PubMed
25. F R Eilber, T T
Grant, D Sakai, et al. “Internal hemipelvectomy: Excision of the hemipelvis
with limb preservation. An alternative to hemipelvectomy.” Cancer, vol.
43, no. 3, pp. 806-809, 1979. View at: Publisher Site | PubMed
26. Gordon P Beadel, Catherine E
McLaughlin, Fawzi Aljassir, et al. “Iliosacral resection for primary bone tumors: is
pelvic reconstruction necessary.” Clin Orthop Relat Res, vol. 438, pp.
22-29, 2005. View at: Publisher
Site | PubMed
27. Joseph
Ippolito, Jennifer Thomson 1, Kathleen Beebe, et al. “Outcomes following
periacetabular tumor resection: A 25-year institutional experience.” J Surg
Oncol, vol. 122, no. 5, pp. 949-954, 2020. View at: Publisher Site | PubMed
28. Xiaodong Tang,
Wei Guo, Rongli Yang, et al. “Acetabular Reconstruction With Femoral Head
Autograft After Intraarticular Resection of Periacetabular Tumors is Durable at
Short-term Followup.” Clin Orthop Relat Res, vol. 475, no. 12, pp.
3060-3070, 2017. View at: Publisher
Site | PubMed
29. Nong Lin,
Hengyuan Li, Weixu Li, et al. “Upshifting the Ipsilateral Proximal Femur May
Provide Satisfactory Reconstruction of Periacetabular Pelvic Bone Defects After
Tumor Resection.” Clin Orthop Relat Res, vol. 476, no. 9, pp. 1762-1770,
2018. View at: Publisher Site | PubMed
30. Tomohiro
Fujiwara, Deepak V Sree, Jonathan Stevenson, et al. “Acetabular reconstruction
with an ice-cream cone prosthesis following resection of pelvic tumors: Does
computer navigation improve surgical outcome?” J Surg Oncol, vol. 121,
no. 7, pp. 1104-1114, 2020. View at: Publisher Site | PubMed
31. Joel L
Mayerson, Adam N Wooldridge, Thomas J Scharschmidt “Pelvic resection: current
concepts.” J Am Acad Orthop Surg, vol. 22, no. 4, pp. 214-222, 2014.
View at: Publisher Site | PubMed
32. Alex Yuen,
Eugene T Ek, Peter Fm Choong “Research: Is resection of tumours involving the
pelvic ring justified? : A review of 49 consecutive cases.” Int Semin Surg
Oncol, vol. 2, no. 1, pp. 9, 2005. View at: Publisher Site | PubMed
33. M E Pring, K L
Weber, K K Unni, et al. “Chondrosarcoma of the pelvis. A review of sixty-four
cases.” J Bone Joint Surg Am, vol. 83, no. 11, pp. 1630-1642, 2001. View
at: PubMed
34. Christian
Delloye, Xavier Banse, Bénédicte Brichard, et al. “Pelvic reconstruction with a
structural pelvic allograft after resection of a malignant bone tumor.” J
Bone Joint Surg Am, vol. 89, no. 3, pp. 579-587, 2007. View at: Publisher Site | PubMed
35. Gordon P
Beadel, Catherine E McLaughlin, Jay S Wunder, et al. “Outcome in two groups of
patients with allograft-prosthetic reconstruction of pelvic tumor defects.” Clin
Orthop Relat Res, vol. 438, pp. 30-35, 2005. View at: Publisher
Site | PubMed
36. Henry J Mankin,
Francis J Hornicek, H Thomas Temple, et al. “Malignant tumors of the pelvis: an
outcome study.” Clin Orthop Relat Res, no. 425, pp. 212-217, 2004 View
at: Publisher Site | PubMed
37. R J Wirbel, M
Schulte, W E Mutschler “Surgical treatment of pelvic sarcomas: oncologic and
functional outcome.” Clin Orthop Relat Res, no. 390, pp. 190-205, 2001.
View at: Publisher Site | PubMed
38. A Kawai, J H
Healey, P J Boland, et al. “Prognostic factors for patients with sarcomas of
the pelvic bones.” Cancer, vol. 82, no. 5, pp. 851-859, 1998. View at: PubMed
39. Muhammad Umar Jawad, Abdul Ahad Haleem, Sean P Scully “Malignant sarcoma of the pelvic bones: treatment outcomes and prognostic factors vary by histopathology.” Cancer, vol. 117, no. 7, pp. 1529-1541, 2011. View at: Publisher Site | PubMed
